Clinical validation of islet autoantibody measurement in self-collected dried blood spot specimens
Primary Objective
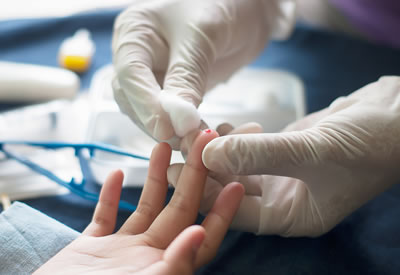
Can ADAP assay accurately detect GADA, IA2A, IAA and ZnT8A in self-collected capillary dried blood spot specimens.
Description
Accessible testing of islet autoantibody may improve the clinical management of type 1 diabetes. We propose to validate the detection of islet autoantibodies for type 1 diabetes in self-collected capillary dried blood spot samples. Capillary blood spots do not require a visit to the doctor office and can be done without a phlebotomist, thereby lowering the testing barrier compared to venous blood draws. The assay performances will be compared to matching serum samples collected from the same individuals tested with FDA-cleared devices. The successful validation of the sample collection kit and assay may lead to the first regulatory-cleared assays for islet autoantibodies in self-collected sample types.
Details
Locations
Barbara Davis Center
Principal Investigator

Kimber Simmons
Study ID
Protocol Number: 23-2561
Categories
Is this Study for You?
Not finding the right Study for you? Join ResearchMatch, a nation-wide registry connecting volunteers and researchers
